Genetic testing has been hailed as helping bring about personalized depression treatment. Theoretically, people may be genetically suited for some specific drugs and less suited for others. We can now guide the choice of antidepressants based on genetic testing. But does that lead to better outcomes?
Not according to a new study in JAMA Network Open. Researchers tested whether an antidepressant choice guided by genetic testing was better than the antidepressant choice with normal clinical judgment. They found that genetic testing did not affect antidepressant efficacy: 24% of the participants “responded” to treatment, and response rates were similar whether they received the testing or not.
“No significant difference in reduction of depressive symptoms was observed,” the researchers write.
That low response rate is particularly striking, given that recent studies have found much higher responses to placebo. For instance, a Cochrane review found a 42% response rate for drug placebo treatment, even for severe depression, and a ketamine infusion trial found a 57.9% response rate for an active placebo.
The research was led by Cornelis F. Vos and Sophie E. ter Hark at the Radboud University Medical Center, Nijmegen, the Netherlands. The study included 56 patients randomly assigned to receive genetic testing-guided antidepressant choice and 55 patients who received treatment as usual (antidepressant choice guided by clinical judgment). All patients received tricyclic antidepressants.
In the genetic testing group, the researchers assessed the patients’ CYP2D6 and CYP2C19 genotypes and determined the starting dose of antidepressants based on these findings.
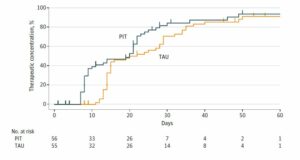
According to the researchers, the primary outcome was whether genetic testing helped the drugs reach “therapeutic plasma concentrations.” They found that about 80% of both groups attained this level. However, those who received a drug guided by genetic testing did reach therapeutic plasma concentrations more quickly: after 17 days rather than 22 days, on average.
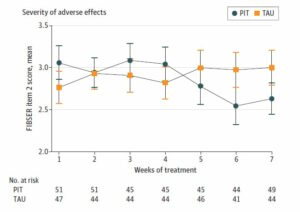
The other outcomes assessed by the researchers were changes in depressive symptoms and adverse effects. The researchers found that genetic testing doesn’t increase the likelihood that the drugs work, with both groups experiencing low response rates. Likewise, the researchers found no difference in adverse effects, on average.
However, they suggest that their study supports the idea that genetic testing can help reduce the harmful side effects of the drugs over time.
In the first four weeks of antidepressant use, those who had antidepressants chosen via genetic testing had more severe adverse effects from the drugs. However, in weeks 5 through 7, those who had genetic testing had less severe side effects. Thus, the researchers conclude that choosing a drug based on genetic testing may reduce the length of time people experience harmful side effects (or reduce the severity over time). However, as the study ended after seven weeks, we can’t see if this pattern holds true for more than those three final weeks.
In sum, the study showed that personalized treatment based on genetic testing did not lead to more improvement in depressive symptoms or fewer harmful effects on average but that there was a potential signal for reduced severity of side effects over time. In addition, the number of people responding to antidepressants was far lower than would be expected, even if the subjects had received a placebo.
What conclusion should we draw from this? The researchers write that their study was a success:
“These findings indicate that pharmacogenetics-informed dosing of tricyclic antidepressants can be safely applied and contributes to personalized antidepressant treatment.”
****
Vos, C. F., ter Hark, S. E., Schellekens, A. F. A., Spijker, J., van der Meij, A., Grotenhuis, A. J., . . . & Janzing, J. G. E. (2023). Effectiveness of genotype-specific tricyclic antidepressant dosing in patients with major depressive disorder: A randomized clinical trial. JAMA Network Open, 6(5), e2312443. doi:10.1001/jamanetworkopen.2023.12443 (Link)
Editor’s Note: Part of MITUK’s core mission is to present a scientific critique of the existing paradigm of care. Each week we will be republishing Mad in America’s latest blog on the evidence supporting the need for radical change.
